
For the best results maintain Sf9 or Sf21 insect cell culture grown on any media in log phase, i.e. in conditions when cells are actively dividing. Do not let suspension culture grow to their highest density and enter stationary phase. Similarly, do not let monolayers overgrow. It is fine if a monolayer grows to confluency and is divided the same day as it reached confluency (confluent monolayer), but do not let a monolayer grow to confluency and divide it a day or two later. A temperature of 27-28oC is optimal for growing of Sf9 or Sf21 cells. In log phase cells grown on TC-100 medium (Sigma, Cat# T3160) supplemented with heat inactivated 10% FBS double in 20-24 h. Cell growth can be slowed by transferring them to r.t. and is a simple method to avoid excessive passage. As the cells do not require CO2 simply put them on the bench. Cells can tolerate temperatures as low as 19oC without adverse effects.
Always pre-warm the media to r.t. when diluting the cells. A maximum recommended cell dilution is 1:10 either from suspension culture with the density 1x106/ml grown on TC-100 medium supplemented with 10% FBS or from the monolayers. However, to be on the safe side, we routinely divide such suspension cultures 1:5 as hemocytometer counts are approximate. Monolayers are handled similarly. For example, if you have 1 flask grown to confluency, you can seed maximum 10 flasks of the same size from that flask. Vent/close flasks are recommended as growth of sparse monolayers is impeded in vent/open flasks and in Petri dishes that allow access of air.
Start cells from the frozen cells stock if you suspect contamination, have overpassaged the cells, have neglected them in stationary phase or you have noticed that they divide slower than before, or do not grow from sparse monolayer (divided 1:10 from confluent monolayer), or if amount of cells that do not attach in the monolayer has increased to over 5-10%.
Religiously maintaining high quality insect cells is crucial for success of the baculovirus expression system and poor quality cell culture is the major course for failure or sub-standard performance. It is much easier to deteriorate an insect cell line than a common mammalian cell line. Therefore, many more researchers achieve top level performance in a mammalian system as compared to relatively few who mastered the baculovirus system.
Clumps can be disrupted by pipetting. However too vigorous pipetting may disrupt the cells, and cell debris may contribute to the precipitation. We apply a more gentle procedure. To get rid of the clumps, leave the culture to settle for several minutes, to the effect that clumps would sediment down to the bottom faster then the individual cells. Take the upper layer containing less clumps and more individual cells, seed a monolayer in a T-flask and leave it on a horizontal surface for about 1 h. Shake the flask to dislodge the clumps and replace the medium. The trick is that clumps are easier dislodged that the individual cells. However, some individual cells will be dislodged also. Therefore, initially you'll need to seed a dense monolayer to have enough attached cells left for the subsequent cultivation.
Clumps problem may have a root in unsatisfactory resuspension of cells derived from a monolayer. Pipette cells derived from a monolayer before starting the suspension culture against the bottom of T-flask. For about 25 ml of resuspended cells pipetting 5-6 times using a 10 ml pipette is sufficient.
Clumps problem could be exacerbated by the presence of particles in the media. Sometimes a clamp could be seen formed around a tiny crystal. The reason is that some components of the media are at saturating concentrations and can easily precipitate out of solution if improperly shipped or stored (temperatures near freezing), or if the medium preparation by the manufacturer was not performed exactly to the specification (components added too fast). Watch for the particles in the media, contact the manufacturer regarding the problem.
L-glutamine in the media decomposes spontaneously into pyrolidone carboxylic acid and ammonia, and its content could be sugnificantly reduced with the storage. Unlike with mammalian media, there is no need to add extra glutamine to the stored insect media. Insect Sf9 or Sf21 cells are not sensitive to the reduced amounts of glutamine and can grow even on glutamine-free media.
You can easily evaluate performance of different media for a particular applications using a GFP reporter. For example, different media can be evaluated for a protein expression application using a Green control recombinant baculovirus which encodes the reporter. There is no need to run protein gels, just infect insect cells growing on different media and compare the green fluorescence. For a transfection application, you can compare performance of different transfection reagents mixed with different insect media using Green Control Plasmid which encodes GFP. Similarly, you can compare green fluorescence of cells transfected at different conditions. There is no need to titrate the virus progeny obtained from different transfection protocol modifications to decide on the one which works best for you.
1. Thaw the serum and pre-warm it to r.t.
2. Place a 100-500 ml bottle of serum into 56oC water bath. Large water bath is preferred Have hot water ready and add it if the temperature in the water bath
drops below 56oC. Make sure that the water level in the water bath is the same or above the level f serum in the bottle. Use a Lead Ring Flask Weight
to prevent flotation of the bottle.
3. Swirl the bottle every 5-10 min to ensure uniform heating of the serum.
4. Incubate 100 ml bottle for 30 min or 500 ml bottle for 35 min.
5. Chill on ice or in cold water bath.
6. Aliquot and store frozen. We routinely make 50 ml aliquots of the serum supplemented with 10x antibiotics. By adding 10x antibiotics into the serum we
economize on future effort, as antibiotics will be added to the medium at 1x concentration when medium will e supplemented with 10% serum.
We routinely heat inactivate Hyclone Standard Serum distributed by Thermo scientific (SH3008803). Typically, its performance is just as good as performance of more expensive sera from the same source. Before ordering, you may like to request free of charge samples of different batches of serum and test them in a protein expression study. Though very rarely, some batches could be sub-standard even from a reliable supplier. We provide a recombinant baculovirus which is ideal for comparison of different batches of sera or different media for protein expression work (Green control). This baculovirus provides for expression of green fluorescent protein (GFP). Therefore, performance of different batches can be assessed simply by comparing intensity of the GFP reporter fluorescence in the baculovirus- infected cells. There is no need to run protein gels. In case you are planning a lot of baculovirus studies, it is a good practice to test several batches and order a large batch of serum to ensure reliable work over a period of 1-3 years.
Thawing insect cells:
1. Prepare a 25 cm2 vent/close flask containing 5 ml of TC-100 medium supplemented with 10% heat-inactivated FBS or FCS and antibiotics (optional). The
medium should be at room temperature. Have a 5 ml pipette, 70% ethanol and tissue paper available.
2. Holding the frozen cells aliquot in your hand, quickly thaw the cells in 37oC water bath by shaking the aliquot until most of the ice is thawed.
Do not allow heating. Do not let the water bath water to touch the screw cap/tube juncture since the most common source of contamination is the
water bath. There is a negative pressure inside the tube; the outside air will rush in as soon as you'll open the tube and will suck in the liquid from the
cap/tube juncture.
3. Wipe the tube dry and then wipe the tube again with tissue paper soaked in 70% alcohol and wipe dry.
4. Using a 5 ml pipette, transfer the cells (~1-2 ml) into the flask and mix them with the medium by gentle pipetting.
5. Leave the flask in a horizontal position at 28oC for 12-24 h. The healthy cells adhere to the plastic. Completely remove
the medium and add 5 ml of fresh medium warmed to room temperature.
6. Continue incubation at 28oC until the monolayer has reached up to 80% confluency. Growing cells to higher confluency generally is not recommended as,
depending on the frozen cell stock, initially cells could be forming a very sparse monolayer. Growing from a sparse monolayer cells form foci in which
cells become very crowded and can divide only on the periphery and stagnate at the center. Harvest the monolayer and transfer the cells into
an 80 cm2 vent/close flask containing 25 ml of the same medium. Incubate until the monolayer is subconfluent. Divide the cells into more flasks, or
start a suspension culture, or aliquot the cells for storage in liquid nitrogen.
Freezing insect cells:
1. Grow Sf9 or Sf21 cells to subconfluent monolayer in 80 cm2 flask on TC-100 medium supplemented with heat inactivated 10% FBS or FCS and
antibiotics (optional). Remove some of the medium, leaving about 10-12 ml in the flask.
2. Harvest the monolayer into remaining medium and add dimethyl sulfoxide (DMSO) to a final concentration of 10 in a cell suspension. Aliquot 1-2 ml of cells into
cryo-tubes (capable of withstanding liquid nitrogen).
3. Place aliquots into a gas phase of the liquid nitrogen tank for at least 2 hours to allow gradual temperature reduction. Alternatively, place the cells into 70-80oC
freezer for about 2 hours, but do not store cells in the freezer for a longer period. Transfer the cells into the liquid phase of the nitrogen storage.
You can seed cells from log phase suspension culture to a monolayer of desirable density any time and leave them on a horizontal surface at r.t. or at 28oC to settle down. It takes up to 1 h for the cells to settle and attach. Cells seeded from suspension culture will grow as monolayers in T-flasks, plates and dishes. They can be used as soon as they attach to the plastic for all types of experiments just as successfully as cells seeded from monolayer to monolayer.
The most common source of contamination is a 37oC water bath that is often used to thaw frozen serum aliquots or warm up the medium. Always wipe dry any container after the water bath, spray it with 70% ethanol and again wipe dry. Add SigmaClean® Water Bath Treatment (Sigma-Aldrich, Cat# S5525) to the water bath to prevent bacterial and fungi growth.
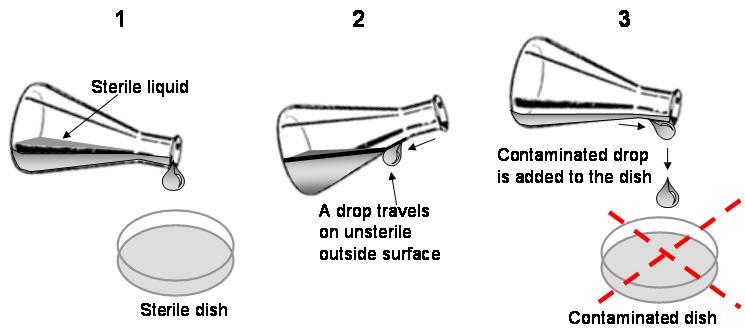
Another common source of contamination is a drop that travels outside a container that is not sterile and ends up in the cell culture. Always be careful not to allow such drops (see below).
Another source of contamination can be a laminar flow cabinet. Remove the grid and the horizontal working surface. Very often you can find dried spilled media, serum and dust that has accumulated over time. Clean it and reassemble. Wipe all the interior of the cabinet with 70% ethanol. Apply a UV lamp. If that does not help, fumigate the cabinet over the weekend. Routinely wipe the working surface with 70% ethanol before you start and after you finish working in the cabinet. Do not store large amounts of disposables (tips, pipettes, plasticware) in the cabinet, put them into the cabinet when needed and remove after you are done. Do not leave the front lid of the cabinet open with airflow not running.
